ethyne lewis structure|C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle : Cebu Lewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it . Watch Live Sex Cams on Dirtyroulette! ️ Free anonymous Adult Cam Chat for random sex with thousands of naked girls and guys.
ethyne lewis structure,A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the total .
ethyne lewis structure C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angleLewis Structure for C2H2 (Ethyne) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it . 399K views 13 years ago. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene). For the C2H4 structure use the periodic table to find the total number of valence.The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. .
In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like C2H2 ---- 1..
The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds .
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the . C2H2 is a chemical formula for Ethyne, a gaseous alkyne hydrocarbon. It has been used widely for welding and cutting purposes. This molecule is also known by the name Acetylene. The compound has a .

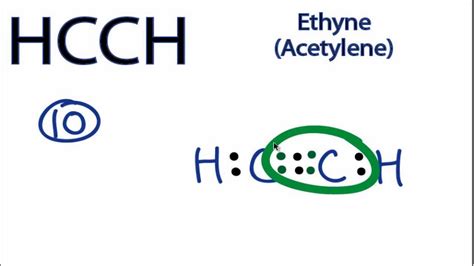
Fundamentals. Bonding in Organic Compounds. Bonding in Ethyne (Acetylene) Expand/collapse global location. Bonding in Ethyne (Acetylene) Page ID. .C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle Fundamentals. Bonding in Organic Compounds. Bonding in Ethyne (Acetylene) Expand/collapse global location. Bonding in Ethyne (Acetylene) Page ID. . draw the structure of alkynes from IUPAC (systematic) and selected common names. Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the .Drawing the Lewis Structure for HCCH. HCCH (Ethyne) can also be written as C 2 H 2. Ethyne is also called Acetylene. For the HCCH Lewis structure you'll need to form a triple bond between the two carbon atoms. Hydrogen atoms only need two electrons for a full outer shell. There are a total of 10 valence electrons for the HCCH Lewis structure. A step-by-step explanation of how to draw the HCCH Lewis Dot Structure (Ethyne).For the HCCH structure use the periodic table to find the total number of val.Lewis Dot of Ethyne (Acetylene) C 2 H 2. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond. Ethene or C2H4 is a common straight-chain acyclic alkene and an important member of organic hydrocarbons. Having a double C=C bond, it is unsaturated and this gives rise to several properties. Here, we learned about how to draw the proper Lewis Structure and find out the molecular geometry of an ethylene molecule.
H. 4. ) Lewis Structure, Hybridization. Ethene's lewis structure can be built by VSEPR rule. Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. C 2 H 2 Lewis structure. C 2 H 2 (acetylene or ethyne) has two carbon atoms and two hydrogen atoms. In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. How to Draw the Lewis Dot Structure for C2H2: .A. Chemical formula of Ethyne. The chemical formula of ethyne is C2H2. It consists of two carbon atoms and two hydrogen atoms. Ethyne is a colorless gas with a distinct odor and is highly flammable. The C2H2 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. II.
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val. Lewis Structure of Acetylene (C2H2) Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation. To study this, first, it is crucial to know the electronic configuration of the participating elements. Carbon (C) has atomic number 6 where its electronic configuration is 1s2 2s2 2p2. Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to .

Ethene is the formal IUPAC name for H 2 C=CH 2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group ( CH2CH3 C H 2 C H 3) but there is a double bond between the two carbon atoms in it. Ethene has the formula C2H4 C 2 H 4 and is the simplest alkene because it has the . Step 3: Connect each atoms by putting an electron pair between them. Now in the C2H2 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms.; Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure. Note that Hydrogen only needs two valence electrons .ethyne lewis structure A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Acetylene (Ethyne)).For the C2H2 structure use the periodic table to find the total n. C2H4 Lewis Structure, Molecular Structure, Hybridization, Bond Angle and Shape. The chemical formula C2H4 represents Ethylene. This molecule is also represented by H2C=CH2, clearly showing the alkene nature of the compound. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C2H4 exists as a colorless gas and is . Ethyne (HCCH), commonly known as acetylene, has a linear structure with a carbon-carbon triple bond. Each carbon (C) atom, with 4 valence electrons, is bonded. . HCCH lewis structure is soluble in both polar and nonpolar solvents but it shows different behavior as it is an organic compound. Apart from that HCCH lewis structure is soluble .
ethyne lewis structure|C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH0 · Lewis Structure for C2H2 (Ethyne)
PH1 · How to Draw the Lewis Structure for C2H4
PH2 · How to Draw the Lewis Dot Structure for C2H2:
PH3 · C2H2 Lewis structure, Molecular Geometry, Hybridization & Bond angle
PH4 · C2H2 Lewis structure, Molecular Geometry,
PH5 · C2H2 Lewis Structure Tutorial
PH6 · C2H2 (Ethyne) Lewis structure
PH7 · C2H2 (Acetylene
PH8 · Bonding in Ethyne (Acetylene)
PH9 · 9.3: Drawing Lewis Structures
PH10 · 1.8: sp² Hybrid Orbitals and the Structure of Ethylene